
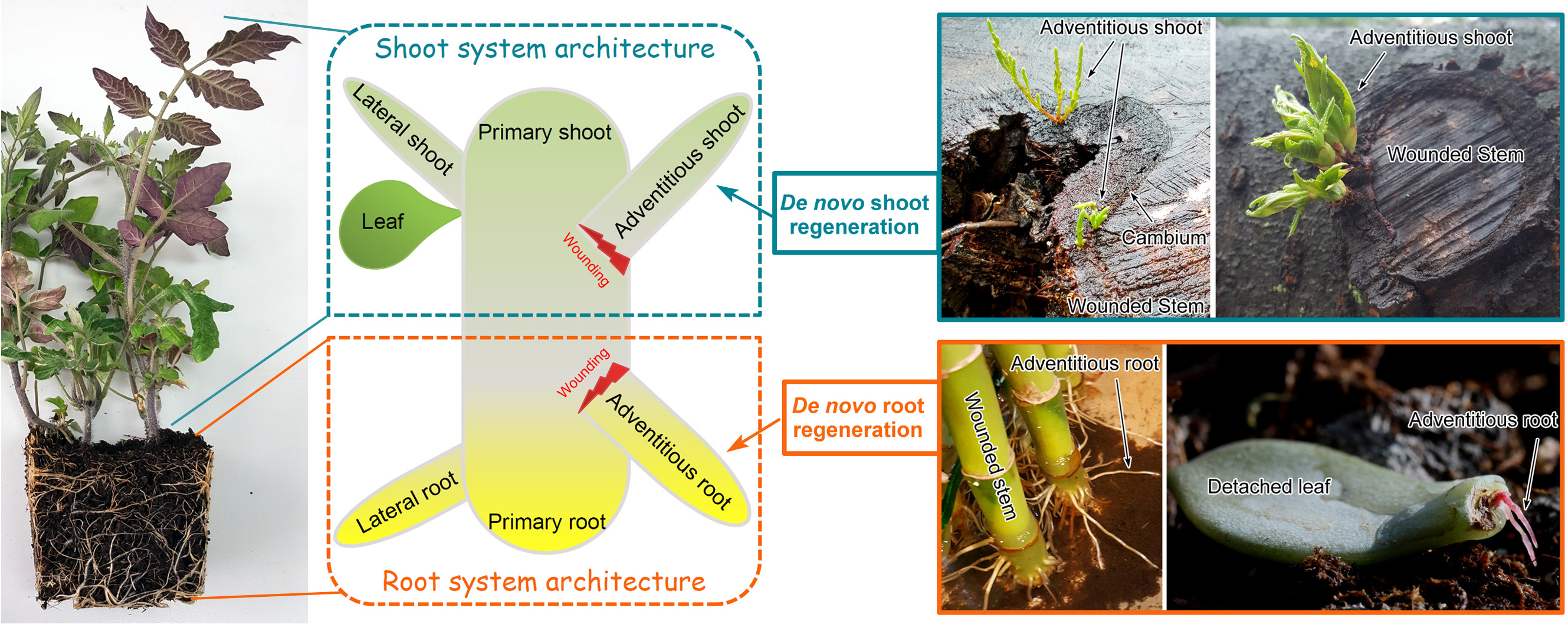
De novo organ regeneration refers to the formation of adventitious shoots (i.e. de novo shoot regeneration) or roots (i.e. de novo root regeneration) after injury. Theoretically, de novo organ regeneration is based on the pluripotency of plant stem cells.
Under natural conditions, de novo organ regeneration allows plants regrow or replenish shoot or root branches, thereby contributing to shoot or root system architecture. Adventitious shoots can regenerate from the cambium region of the wounded stem after trimming of the tree crown. Adventitious roots can regenerate at the bottom wound of a stem or from detached leaves, such as root formation in cuttings.
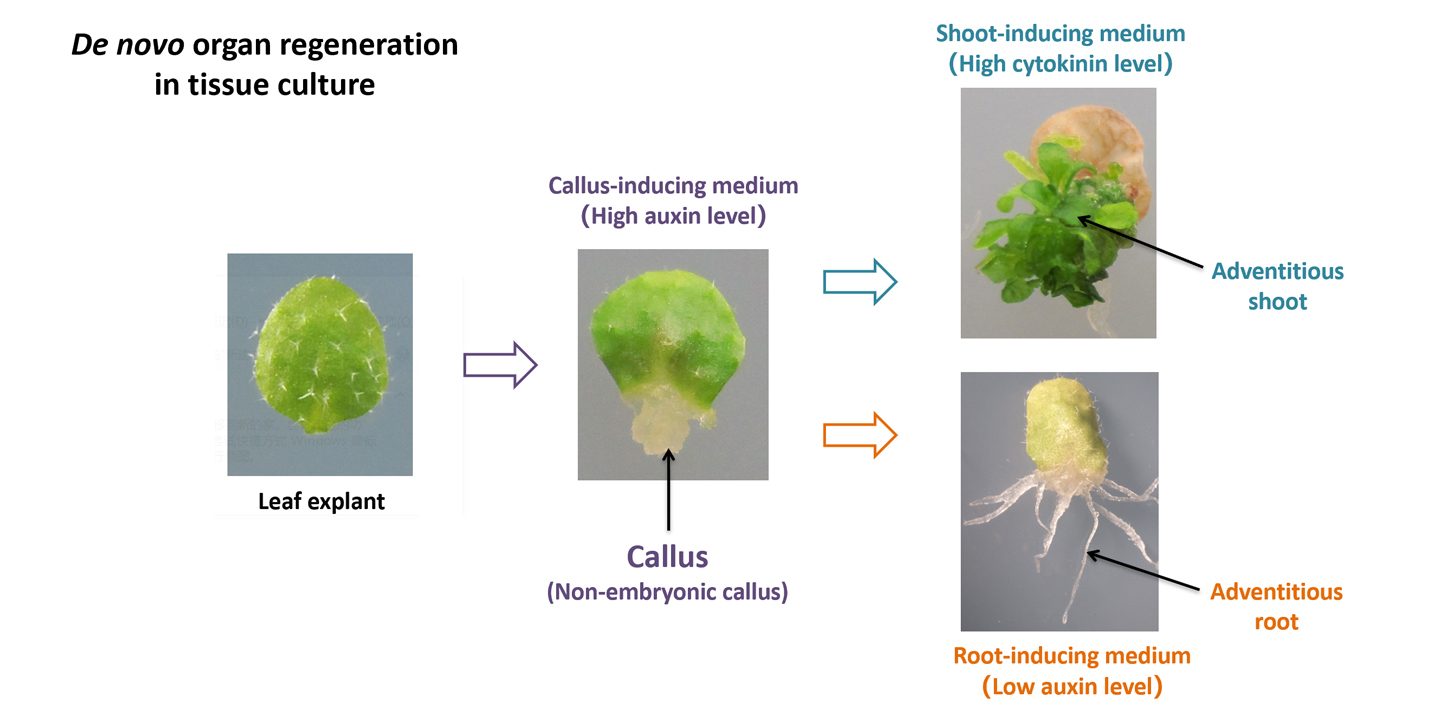
In in vitro tissue culture, the ability to regenerate adventitious roots/shoots has long been exploited for vegetative propagation. In the 1950s, Skoog and Miller found that different concentrations of auxin and cytokinin in the medium were the key to induce in vitro organogenesis (Symp Soc Exp Biol, 11:118-130, 1957). This discovery laid the foundation for the exploitation of organogenesis in tissue culture and was considered to be one of “the scientific roots of modern plant biotechnology” (The Plant Cell, 20:1189-1198, 2008). The two-step tissue culture technology is now widely used in agriculture. In the first step (i.e., pluripotency acquisition), the pluripotent non-embryonic callus is formed by a high level of auxin in the callus-inducing medium; and in the second step, (i.e., organogenesis), adventitious shoots can be formed from callus by a high level of cytokinin in the shoot-inducing medium, or adventitious roots can be formed from callus by a low level of auxin in the root-inducing medium.
The overall goal of our research is to reveal the molecular basis and evolutionary route of stem cell pluripotency that allows plants to regenerate adventitious roots and shoots. We have three research directions: de novo root regeneration in leaf cuttings, pluripotency of callus in tissue culture, and evolution of plant stem cells and organs.
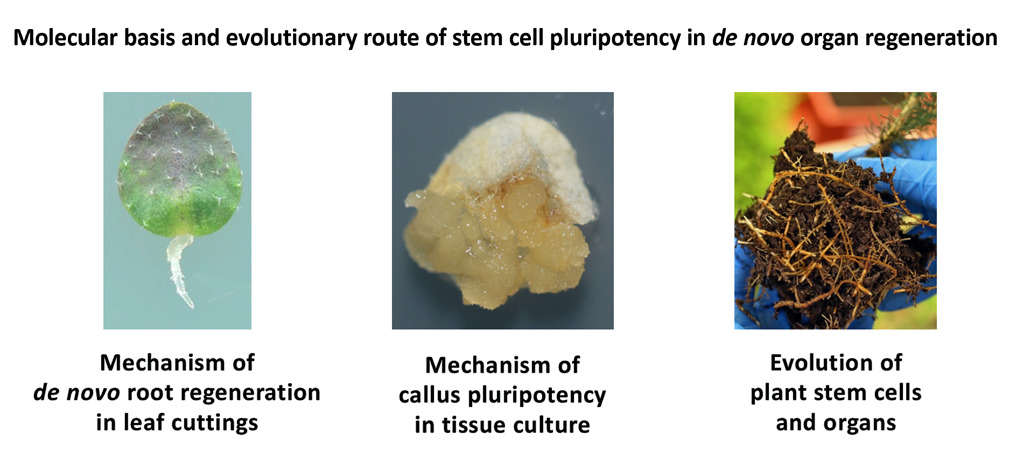
De novo root regeneration in leaf cuttings. Detached Arabidopsis leaves are cultured on B5 medium without exogenous hormones, and adventitious roots can be regenerated from the wounded site on the detached leaf. Detached leaves sense many early signals (wound signals, environmental signals, and developmental status) to adjust auxin production. Auxin is then transported into regeneration-competent cells in the vasculature and triggers cell fate transition to form roots. We aim to reveal the mechanism from signal sensing to cell fate transition during de novo root regeneration in leaf cuttings.
Pluripotency of callus in tissue culture. We use the in vitro tissue culture system to study callus formation and organ regeneration. Non-embryonic callus is a group of pluripotent cells that can regenerate either shoots or roots. We aim to reveal the mechanism of pluripotency acquisition in callus.
Evolution of plant stem cells and organs. We have collected typical model plants (Marchantia polymorpha, Selaginella kraussiana, Psilotum nudum, Ceratopteris richardii, rice, and etc.) to study the evolution of plant stem cells and organs. Currently we are focusing on analysis of how roots had been originated and evolved in vascular plants.